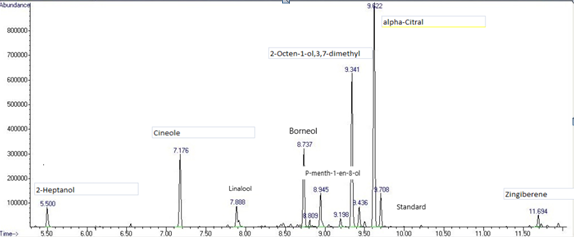
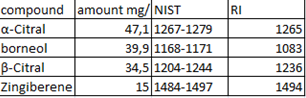
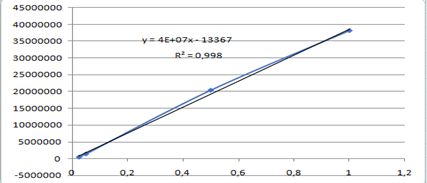
Ginger, which is a rhizome plant, is known to have a very strong smell and is often used in our food for example when we prepare rice or any other sauce to give it a spicy edge and a sweet smell. Many people use it to make tea or herbal teas, grating or chopping it and mixing it with boiling water, and it goes well with lemon. Over the years, we've discovered that it's not only used for culinary purposes, but also has many therapeutic properties. When you're feeling sick to your stomach, drinking an infusion of ginger can help reduce heartburn. Studies show that ginger supplements are effective in reducing inflammation in the body, as well as helping with digestive problems. [2]
[1] = Britannica, T. Editors of Encyclopaedia. "ginger." Encyclopedia Britannica, May 29, 2023. https://www.britannica.com/plant/ginger.
[2] = Zingiber officinale Roscoe in Döring M (2022). English Wikipedia - Species Pages. Wikimedia Foundation. Checklist dataset https://doi.org/10.15468/c3kkg
Nist web book : https//webbook.nist.gov/chemistry/