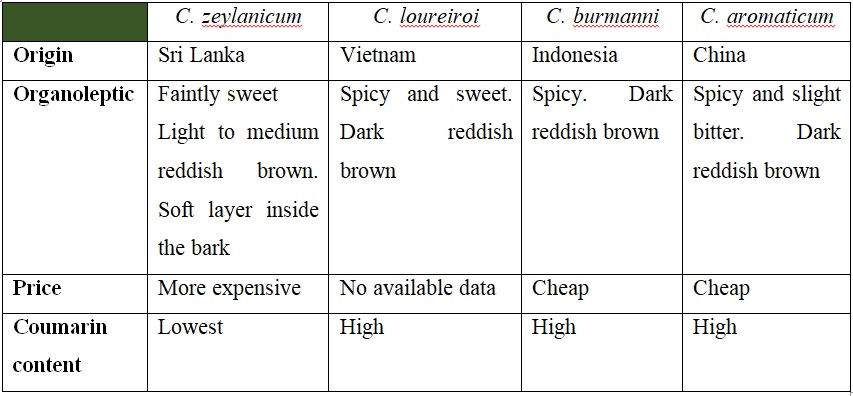
From the above table, it shows that the difference amount of coumarin in the cinnamon bark. The cinnamon bark with lowest coumarin content is cinnamon bark originated from Sri Lanka. High coumarin consumption has been linked to negative effects to health. It was first thought that coumarin can cause genotoxic and carcinogenic effect. However, the study showed that high consumption of coumarin can cause liver damage (hepatotoxic) (Jeremic, et al., 2019)

Cinnamon Bark Hydrosol
Cinnamon bark is cultivated from the tree of Cinnamon, which is distributed widely in Asia and Africa. The tree can grow approximately up to 15 m and posses highly aromatic bark and leaves (Charles, 2013). There are four species of cinnamon that are economically important, namely Cinnamomum zeylanicum Blume (known as Sri Lanka cinnamon), Cinnamomum loureiroi Nees (known as Vietnamese cinnamon), Cinnamomum burmanni (known as Indonesian cinnamon), and Cinnamomum aromaticum Nees (known as Chinese cinnamon or Cassia cinnamon) (Kawatra & Rajagopalan, 2015). Cassia cinnamon is the most common cinnamon bark found in the grocery shop and the price is lower than Sri Lanka cinnamon (Leech, 2023). The table below summarizes the characteristics of the four cinnamon barks that will be helpful to screen the cinnamon barks (Kawatra & Rajagopalan, 2015; Leech, 2023):
Preparation of Cinnamon bark hydrosol
Cinnamon bark hydrosol was created using the microwave extraction method. The cinnamon sticks were cut into small pieces and then blended to obtain cinnamon bark grounded powder. 300g of cinnamon bark powder was placed in the microwave extraction vessel and then, 0.5L of boiling distilled water was added. The cinnamon bark powder was left soaked in the water for overnight. The next day, a 300mL beaker was placed in the middle of the microwave vessel. An ice cone was then screwed onto the lid of the extraction vessel, and the entire vessel was placed into a normal microwave, along with a microwave safe mug filled with cold water. The microwave was set to run for a period of nine minutes. After the 9 minutes is up, the vessel was then removed from the microwave and the cinnamon hydrosol was collected. The beaker is then replaced and a new ice cone is fitted to the vessel lid, at which point the process repeats. The microwave extraction process was repeated for 3 times to ensure all volatile and water soluble components from the cinnamon bark powder were extracted.

Analysis of Hydrosol
Prior the analysis using GC-MS instrument, 1 mL of hydrosol sample was filtered through SPE (solid phase extraction) to remove the water and large impurities that might clog the GC column. GC-MS analysis is useful to identify and quantify the components that are present in the cinnamon bark hydrosol.
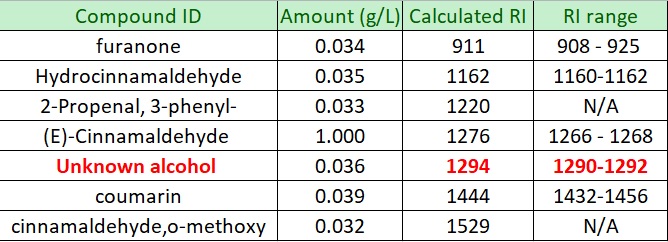
The qualitative analysis to identify each peaks in the chromatogram was performed using the library search and by comparing the calculated Retention Index (RI) values with the RI values obtained from NIST website. In addition, the concentrations for each component were calculated by using the internal standard (o-guaicol) calibration curve.
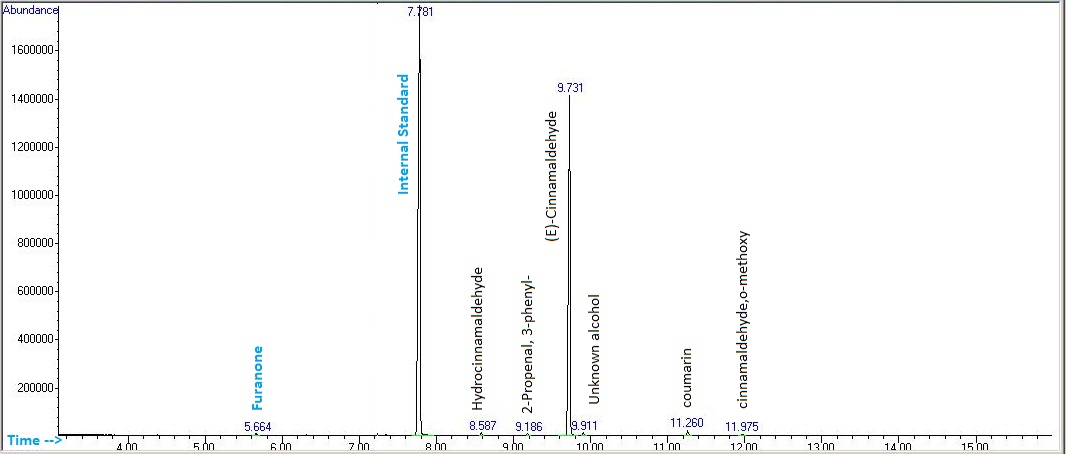
Major component in the cinnamon bark hydrosol
From GC-MS analysis, it is observed that Cinnamaldehyde is the major component in the cinnamon bark hydrosol. This finding is in line with the literature that stated Cinnamaldehyde as the major component (60 – 80%) in the cinnamon bark. It was investigated that cinnamaldehyde which can be found in the essential oil and water extract of cinnamon bark, exhibits various medicinal properties, for instance anti-inflammatory, antioxidant, antitumor, anti-diabetic, COX-2 inhibition, antiproliferative against human colon cancel cells, and antibacterial against various types of bacteria (Charles, 2013; Nabavi, et al., 2015; Ziegenfuss, Hofheins, Mendel, Landis, & Anderson, 2006; Hong, et al., 2012; Doyle & Stephens, 2019)
References
Charles, D. J. (2013). Antioxidant Properties of Spices, Herbs and Other Sources. New York: Springer .
Doyle, A. A., & Stephens, J. C. (2019). Areview of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia, 1-18.
Hong, J.-w., Yang, G.-E., Kim, Y. B., Eom, S. H., Lew, J.-H., & Kang, H. (2012). Anti-inflammatory activity of cinnamon water extract in vivo and in vitro LPS-induced models. BMC Complementary and Alternative Medicine, 1-8.
Jeremic, K., Kladar, N., Vucinic, N., Todorovic, N., Hitl, M., Lalic-Popovic, M., & Gavaric, N. (2019). Morphological characterization of cinnamon bark and powder available in the Serbian market. Biologia Serbica, 89-93.
Kawatra, P., & Rajagopalan, R. (2015). Cinnamon: Mystic powers of a minute ingredient. Pharmacognosy Research, S1-S6.
Leech, J. (2023, February 1). Ceylon vs. Cassia — Not All Cinnamon Is Created Equal. Retrieved from https://www.healthline.com/nutrition/ceylon-vs-cassia-cinnamon#TOC_TITLE_HDR_1
Nabavi, S. F., Lorenzo, A. D., Izadi, M., Sobarzo-Sanchez, E., Daglia, M., & Nabavi, S. M. (2015). Antibacterial Effects of Cinnamon: From Farm to Food, cosmetic, and Pharmaceutical Industries. Nutrients, 7729-7748.
Ziegenfuss, T. N., Hofheins, J. E., Mendel, R. W., Landis, J., & Anderson, R. A. (2006). Effects of a Water-soluble cinnamon extract on body composition and features of the
I am Juli Jayanti and currently I am completing a Master's program in Instrumental Chemical Analysis at Trent University. Throughout my studies, I have obtained extensive trainings and hands on experiences in analytical chemistry using various instruments, such as ICP-MS, GC-MS and LC-MS. It is a great opportunity to work as a summer placement student at the John L. Holmes Mass Spectrometry Facility – University of Ottawa, where I can further develop and gain more practical experiences in analytical chemistry.
