- Autonomous Reactor Platform
- IoT-enabled Reactor for Continuous Manufacturing
- Continuous Process Validation
- Space-Time Reactor

In process chemistry, batch failure is an inconvenient truth that we all learn to cope with. No matter how robust a process is, at some point in the lifetime of a production campaign batch failure is likely to occur. Unfortunately, we can't trace time back and situate ourselves at a chronological time before the batch failed. In batch manufacturing, the state of a reactor (i.e, the reagent to product distribution ratio) of the present cannot be set back to a past clock time. Let's consider an example.
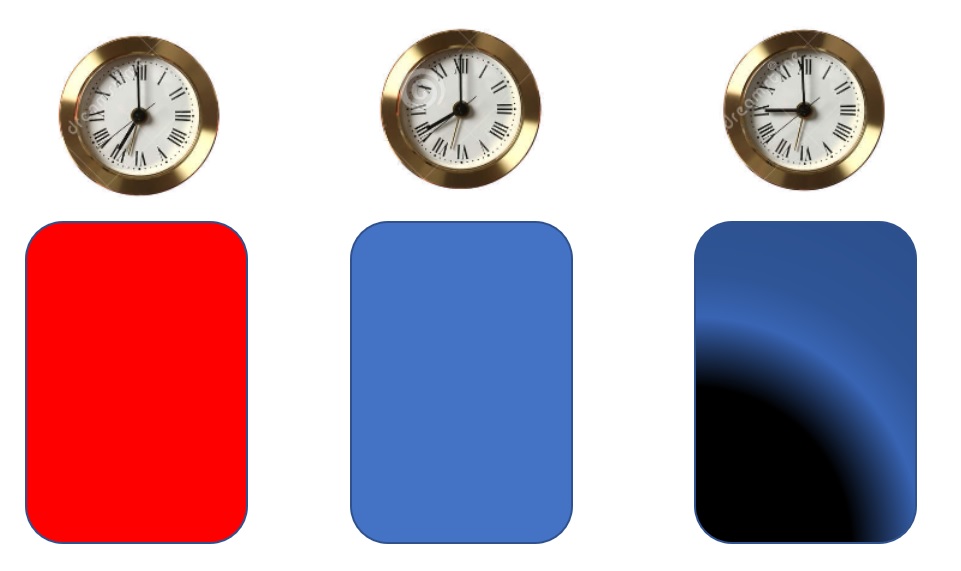
Let's imagine a batch process that uses reagent red to give product blue at 7'o clock as shown in Fig 1. By 8'o clock, most of the red reagent has converted into the blue product. The situation becomes interesting if the product is not fished out of the batch vessel at the 8'o clock mark. By 9, a byproduct (shown in black) starts to form. Unfortunately, if we are at the 9'o clock mark, we can't take the reactor 'back to the future' and get back all good product of the 8'o clock mark.
Now let's consider what happens when the same experiment is done in a flow format. In flow, reagent molecules (for example, the reagent red) enters a flow tube and exits as the red product molecules in an orderly manner (Fig 2). The time a molecule spends in the energized reaction tube is predictably correlated by the tube length. The picture below illustrates the reaction time/reactor length relationship using our red regent-blue product example. In this case, the reagent red is flowed from left to right through an energized reactor tube. As the reagent travels across the tube length, the reagent starts to form the product. If you wait too long (i.e., flow the reagent slowly over two chronological hours), you might even see some of the black side-product coming out at the end of the reactor tube. You can do this experiment any time of the day. The experiments at the 7'o clock, or at the 8'o clock, or at 9 are expected to give the same reactor state.

This gives rise to a unique advantage in doing synthesis in flow. Because the time-dimension is converted into a length-dimension, we gain the ability of modulating reactor states at various time-dimensions by manipulating effective reactor lengths. If we are solely witnessing the evolution of the red reagent into the blue product overtime for our 7'o clock flow experiment, we see our 7'o clock batch reactor state at the start of the flow reactor tube, the 8'o clock batch reactor state somewhere in the middle of the flow reactor tube, and the 9'o clock state at the end of the same flow tube (Fig 3). As long as we have a device that informs us where these chronological batch reactor states are across the length of the flow tube, we can channel matter out of the tube on time and save a batch (or the experiment) from being spoiled.
Prof. Michael Organ, an expert of flow chemistry from the University of Ottawa, and Prof. Jeff Manthorpe, an expert of organic synthesis of Carleton University, joined hands to harness this unique advantage of flow chemistry into a space-time reactor initiative. The Flow Research Facility, in collaboration with a major fluid diverter company, designed and manufactured the prototype of a unique fluid diverting device that allowed the space-time reactor team to monitor a flow process not only over (chronological) time, but also across the reactor length as a reagent residence time convertible. To learn more, or to find out if you can take advantage of our space-time reactor for your application, please contact us.
